

3 He deduced (but does not give a calculation to show how) that the rays consist of a particle beam with m/ e ratio of 3.2 × 10 -3 'CGS units'. Using a magnetic field of 3250 'CGS units' (probably gauss) and a electrical field of 2000 V, applied in the same direction of the magnetic field, Wien's positive ray showed a deflection of 6 mm. Using very powerful electromagnets, he observed that the canal rays deflected in a direction opposite to the cathode rays, which implied they carried a positive charge of electricity, and this led them to be renamed as 'positive rays'. One year later, in 1898, Wilhem (Willi) Wien, while working as an assistant to Hermann Helmholtz in Berlin, was inspired by Thomson's work and began his own investigations into cathode and canal rays. Source: © National Library of Congress/SPL

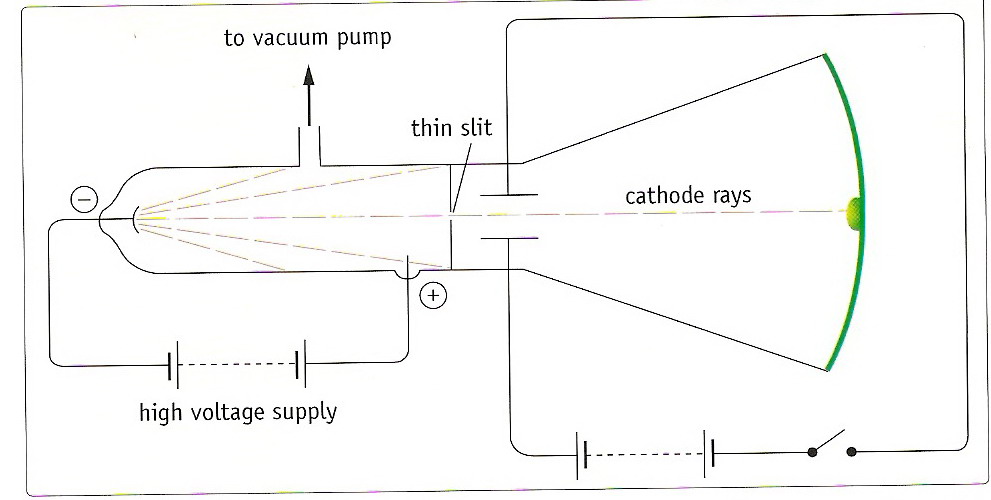
Using the strongest magnet he had, one that certainly had an effect on the cathode rays, Goldstein attempted to deflect his canal rays, but he observed no change in path. 2a Unlike the cathode rays, the colour of the canal rays depended on the identity of the residual gas in the tube, though the significance of this observation was not appreciated at the time. On account of their appearance, Goldstein named them Kanalstrahlen, which means 'canal rays'. If the cathode was relatively thick, they would appear as a parallel beam. Some years later, in 1886, the German physicist Eugen Goldstein noticed that if he used a perforated cathode, then in addition to the cathode rays between the anode and the cathode, there was a different type of ray emerging from the holes in the cathode, and moving in the opposite direction to the cathode rays. 1 This was early evidence of 'cathode rays', though nothing was known of their properties. In 1858, professor of physics at the University of Bonn, Julius Plücker, while investigating the action of a magnet on the electric discharge of rarefied gases, observed a cathode-induced green fluorescence on the glass walls of a discharge tube. The phenomenon began to receive rigorous scientific study in the mid-19th century with the advent of induction coils that could provide high voltages sustained over long periods of time and the availability of high capacity batteries to power the induction coils. That coil also keeps the electron in the centered path.At the end of the 18th century experimental scientists were aware that a spark generated electrostatically could travel longer distances in a partially evacuated glass tube than in air. Note a negatively charged cylinder over the coil.

Now $e/m$ is a huge number for electrons, and $V$ is large, so you can imagine that the electrons are directionally moving with a very very high velocity. If the acceleration voltage different between the anode and the cathode is $V$, you can determine the velocity of electrons $v$ as This virtual experiment shows that the single hole in this diagram is indeed positively charged and one may ask why electron just get absorbed in it? The key point that you remember is that the voltage difference, although it looks like a small battery, is actually several thousand volts. The "electrons" (unknown at that time), were studied with tubes like these ( ): The electron was not discovered with the perforated cathode experiments. In the canal experiments, positive charges pass through the hole when there is a reasonable number of gas particles. I gather you are talking about the canal ray experiment rather as shown in the picture.


 0 kommentar(er)
0 kommentar(er)
